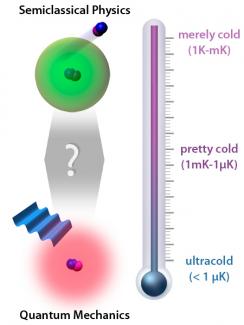
Understanding how molecules collide is a hot topic in ultracold physics. Knowing the number of times molecules crash into each other and what happens when they do helps theorists predict the best ways to cool molecules to merely cold (1 K–1 mK), pretty cold (1 mK–1 µk), or ultracold (< 1 µK) temperatures.
Fellow John Bohn recently decided to investigate the general collision behavior of polar molecules in low-temperature gases. He wanted to see whether knowing the molecule’s dipole moment and mass would be sufficient to predict the size of its "sphere of influence," a.k.a. the scattering cross section. Think of it as a cage surrounding a polar molecule that delineates the boundaries of its interactions with other polar molecules. To figure all this out, Bohn collaborated with two former JILAns, Mike Cavagnero (University of Kentucky) and Chris Ticknor (a postdoc at Australia’s Swinburne University of Technology).
Bohn’s analysis yielded some interesting results. At ultracold temperatures, the molecular dipole system could be readily modeled using some simplifying assumptions in quantum mechanics. He found that all polar molecules would behave in pretty much the same, predictable way under ultracold conditions. He also discovered that the size of the sphere of influence was indeed determined mostly by the dipole moment. Moreover, the sphere of influence would be large. Even weak distant forces could have a large effect on individual molecules. Since molecules move very slowly at ultracold temperatures, there would be plenty of time for interactions, too. Even so, it isn’t clear whether chemical reactions would take place because the kinetic energies of ultracold polar molecules are so low.
At merely cold temperatures, the system could also be readily modeled, this time with semiclassical approximations. Again, any polar molecule would behave pretty much the same way as any other under the same circumstances, once allowances for different dipole moments were taken into account. However, the spheres of influence would be much smaller, and the molecules would move quite a lot faster. Because they are traveling faster, the molecules would interact weakly, and head-on collisions would be rare. Though rare, collisions could cause chemical reactions.
In contrast, at pretty cold temperatures (the terrain between the merely cold and the ultracold), collision details vary from one molecule to the next, and there is no single, catchall theory that applies universally. What happens at these transition temperatures is anyone’s guess at the moment. Luckily for theorists, experimental data will soon appear for pretty cold polar molecules. By mining this data, Bohn and his colleagues hope to be able to construct a more complete theory to explain them.
"In their experiments with KRb molecules, the Ye and Jin groups are observing collisions in the 1 mK–1 µK range," Bohn said. “"We hope to one day tell them exactly what they are seeing." - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.